SPONSOR/ CRO INFORMATION
Thank you for your interest in our dedicated clinical research and vaccine center, Clinical Research Professionals. We are dedicated to the ethical and professional management of clinical trials, with integrity and quality being a leading value. Our team members are trained medically and hold multiple certifications. Clinical Research Professionals has dedicated and experienced specialists for regulatory, recruitment, budgets and contracts in addition to our clinical team. A regulatory submission can be completed in as little as 3 days. Our average contract and budget timeline is 7-10 days. Our dedicated recruitment department assures successful enrollment of appropriate study participants. We are committed to excellence.
|
Please click this button to fill out the contact request form and one of our clinical research professionals team members will contact you. We look forward to working with you for all of your research needs.
|
|
EXPERIENCE
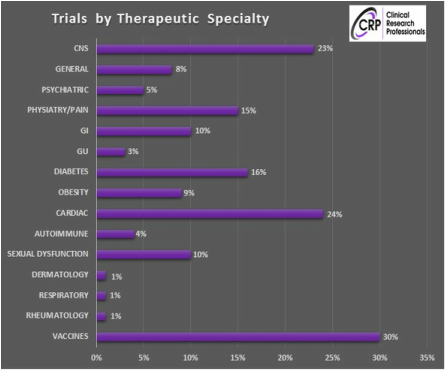
Our experience includes phases 2-4 in the following therapeutic areas |
|
Migraine Headaches
Alzheimer’s Disease Memory Impairment Restless Leg Syndrome General Anxiety Disorder Depression Gastroparesis EoE (Eosinophillic Esophagitis) Hyperlipidemia Hypertension Coronary Artery Disease Diabetes – Type I & II Obesity Osteoarthritis Pain Male Sexual Dysfunction Female Sexual Dysfunction Overactive Bladder Heartburn IBS NASH Nocturia Ulcerative Colitis Traumatic Brain Injury Vaccines Parkinson's Disease |
We look forward to an opportunity to work with you and your team to help you achieve your objectives and exceed your goals.
|
|
|
To View Our Clinical Trial Experience Click Here
|